Updates from the Advisory Committee on Immunization Practices February Meeting
Mar 06, 2018

Photo Credit: James Gathany, Centers for Disease Control and Prevention
The Advisory Committee on Immunization Practices (ACIP) held its first meeting of 2018 on February 21st and 22nd. The Committee consists of a panel of immunization experts that advise the Centers for Disease Control and Prevention (CDC). Part of their charter is to continually evaluate new data and update or change vaccine recommendations as warranted.
The agenda for the February 2018 meeting included presentations pertaining to several different diseases and vaccines, to include hepatitis, influenza, anthrax, HPV, pneumococcal, meningococcal and Japanese encephalitis.
A overview of the meeting is provided below, with details on presentations in the order they occurred:
Hepatitis B
The committee voted unanimously to approve a non-preferential recommendation for a new Hepatitis B vaccine (Dynavax’s HEPISLAV-B™) to their list of recommended vaccines for adults 18 years and older against infections caused by all known subtypes of Hepatitis B.
This vote came following the presentation of data showing that the new two-dose vaccine generates a more rapid and higher antibody response than the standard 3 dose vaccine.
Hepatitis B is a viral disease of the liver that can become chronic and lead to cirrhosis, liver cancer and death. The hepatitis B virus is 50 to 100 times more infectious than HIV, and transmission is on the rise. In 2015, new cases of acute hepatitis B increased by more than 20 percent nationally and 850,000-2.2 million persons are estimated to be living with infection in the U.S.
Since there is no here is no cure for hepatitis B, vaccination is our best chance at preventing the disease. While about 90% of people are infected during infancy, in adults, hepatitis B is most often spread through contact with infected blood and through unprotected sex with an infected person. Some individuals who are especially susceptible include those who are immunosuppressed or living with diabetes. The CDC recommends vaccination for those at high risk for infection due to their jobs, lifestyle, living situations and travel to certain areas.
The Working Group summary suggested that this new vaccine option is likely to improve vaccine series completion and result in earlier protection, which is especially beneficial in persons with anticipated low adherence such as injection drug users. Additionally, the improved immunogenicity in populations with typically poor vaccine response such as the elderly, diabetics and those on dialysis, is promising. The ACIP will continue to review post-marketing surveillance studies and additional data to ensure safety and cost-effectiveness considerations.
Hepatitis A
The committee voted unanimously to pass three recommendations pertaining to Hepatitis A.
- Hepatitis A vaccines should be administered for post-exposure prophylaxis for all persons 12 months of age or older.
- Hepatitis A vaccine or immune globulin (IG) may be administered to persons 40 years of age or older, depending on the providers’ risk assessment.
- Hepatitis A vaccine should be administered to infants age 6-11 months of age traveling outside the US when protection against hepatitis A is recommended. This recommendation takes into consideration the fact that infants under 12 months who will be traveling internationally will typically also need an MMR vaccine. Since Hepatitis A immune globulin and MMR vaccine should not be administered simultaneously, these children should receive a single dose of HepA vaccine. It’s important to note that infants should then complete the full, 2 doses of MMR and HepA vaccines at 12 months of age as recommended.
Influenza
The Committee heard five presentations specific to influenza.
The first two were reports of current season data; one detailing flu surveillance, the other providing early influenza vaccine effectiveness data.
According to the update, the majority of circulating flu strains are similar to those contained in the 2017-2018 vaccine. The only virus clearly showing antigenic drift was the B/Victoria lineage viruses which represents less than 1% of circulating viruses. So far this season, influenza A (H3N2) has been dominant, with influenza B activity starting to increase more recently. Activity has been the highest we’ve seen since 2009, and while final severity can’t be determined until the end of the season, hospitalization rates and mortality could be similar to or exceed those send during the severe 2014-2015 season.

Based on data from 4,562 children and adults with acute respiratory illness enrolled during November 2, 2017–February 3, 2018, at five study sites, the overall estimated effectiveness of the 2017–18 seasonal influenza vaccine for preventing medically attended, laboratory-confirmed influenza virus infection was 36%. The percentage differs by age group and by virus. A detailed report can be found here.
The most notable news out of the Committee last week was the vote to restore the live attenuated influenza virus (LAIV) vaccine as an option for the 2018-19 season. LAIV is commonly known as the nasal spray flu vaccine or by its brand name, FluMist This renewed ACIP recommendation offers FluMist as one of several vaccine options for non-pregnant people who are 2-49 years of age during the 2018-2019 season, but does not indicate any preference for FluMist over injectable flu vaccines.
While FluMist has not been recommended for the past two flu seasons due to reduced effectiveness against the H1N1 flu strain in children, the Committee heard three presentations specific to LAIV vaccine efficacy in children prior to taking a vote on future recommendations for LAIV. The first reported on the efficacy of Fluarix Quadrivalent in children 6-35 month of age. Another presented the results of a randomized trial of a new H1N1 LAIV strain in U.S. children. The third was a review LAIV in children 2-17 years of age.
The possible root cause of the poor effectiveness of LAIV against H1N1 was discussed and poor replication of the H1N1 selected strain was thought to be the likely problem. A new strain selection process is now in place in cooperation with the Food & Drug Administration (FDA) and it suggested that the antibody responses of the latest reformulated version of the quadrivalent vaccine, which includes the new 2017-18 post-pandemic 2009 H1N1 LAIV strain (A/Slovenia), will perform significantly better than what was previously observed when the vaccine included the 2015-16 post-pandemic LAIV strain (A/Bolivia). Immunogenicity and viral shedding data in small trials supported this notion, but no efficacy data is available at this time.
The Committee was therefore forced to a vote using only the science available to date. There was a lively discussion among members who expressed various concerns. While flu vaccine effectiveness is a serious issue, some committee members expressed concern that they may be holding FluMist to a higher standard than other influenza vaccines, yet all have efficacy challenges from year to year. Other members were concerned with how the vaccine may perform in an H1N1 dominated season. Until the vaccine is used, further effectiveness assessments are performed, and a prominent H1N1 year occurs, a certain level of uncertainty will remain.
While members voted overwhelmingly (12-2) to reinstate LAIV on the immunization schedule, a second vote to give other flu vaccines a preferential recommendation over LAIV failed (11-3). So, while the ACIP will not indicate a preference for any one type of flu vaccine over another, the public will ultimately determine whether there will be high uptake of this particular vaccine next season.
Evidence to Recommendation
The Committee voted unanimously to adopt an “Evidence to Recommendation” framework that will be used for future decision making purposes and will include the indirect benefits of vaccination as a consideration of Benefits and Harms.
Anthrax
The Anthrax Working Group presented findings from studies that evaluated the best route of administration and dose-sparing strategies for the anthrax vaccine during large-scale anthrax events.
HPV
The Committee heard five presentations specific to HPV.
The first two were reviews of the vaccine safety data from VAERS and the Vaccine Safety Datalink which are used to assess risk of any adverse events after receipt of 9vHPV vaccine.
The VAERS data covered reports received during the time period from Dec 1, 2014 through Dec 31, 2017. There were a total of 7,244 reports and 97% were non-serious. The most frequent adverse events were dizziness, syncope (fainting), headache and injection site reactions. There have been approximately 29 million 9vHPV does distributed in the U.S. and no new safety signals or unexpected patterns were observed. The safety profile remains consistent with data from pre-licensure trials and post-licensure data on 4vHPV, but both the CDC and the FDA will continue to monitor and evaluate the safety of 9vHPV.
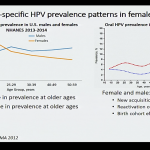
A third presentation discussed the need to harmonize the upper age recommendation for HPV vaccination among females and males.
It was noted that in 2009 the FDA licensed the quadrivalent HPV vaccine for use in males age 9-26 for the prevention of anogenital warts. Due to uncertainty about the impact and cost effectiveness, and the desire for more efficacy data against anal pre-cancers in males, the ACIP guidance provided at that time was that the quadrivalent vaccine may be given to males 9-26 years of age.
By October 2011, the ACIP concluded that the burden of disease in males justified routine vaccination and that vaccinating both males as well as females was not only cost-effective, but would protect heterosexual males and their female sex partners, as well as men having sex with men. However, higher costs for vaccinating 22-26-year-old males remained a consideration for limiting the age recommendation for males at that time.

After the 9-valent HPV vaccine was licensed by the FDA for both females and males in 2014, the ACIP recommended vaccination of males and females with 9vHPV in 2015. However, the current recommendation is not consistent among males and females in the upper ages.
Currently, the HPV vaccine recommendations read as follows:
•HPV vaccine is recommended for routine vaccination at age 11 or 12 years. (Vaccination can be started at age 9.)
•ACIP also recommends vaccination for females aged 13 through 26 years and males aged 13 through 21 years not adequately vaccinated previously.
•Vaccination is also recommended through age 26 years for gay, bisexual, and other men who have sex with men, transgender people, and for immunocompromised persons (including those with HIV infection) not adequately vaccinated previously.
The ACIP would like to consider harmonizing the age recommendations for males and females, which is why the ACIP began reviewing the burden of disease and HPV epidemiology in males during this meeting. They will continue to evaluate vaccine efficacy data in males during future meetings in anticipation of a potential vote in 2018.

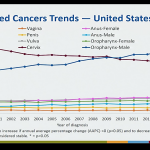
The next HPV presentation specifically examined trends in HPV-associated cancer types in the U.S. population from 1999-2014, which was followed by a review of the burden of disease in males.
Some of the interesting data points that were mentioned included:
- There were 34,864 cases of HPV cancer from 1999-2014; 62% among females and 38% among males
- A rise in incidence of oropharynx cancers, increasing from 6,850 cases in 1999 to 14,931 cases in 2014 (which quite possibly can be due to better surveillance).
- Men have higher rates of oral HPV infections but it is unknown how these infections become cancer.
- There is no screening test for HPV related head and neck cancers.
- Oral HPV prevalence is lower in men who have received the HPV vaccine.
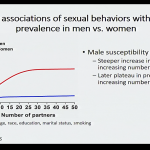
- Data suggests that there is a steeper increase in prevalence of oral cancer among males who have a higher number of sexual partners than what was observed among women.
Pneumococcal
In 2014, the ACIP recommended the use of both PCV13 and PPSV23 in adults age 65 years and older. At that time, it was discussed that the ACIP should re-evaluate the decision to routinely use PCV13 among adults in an attempt to distinguish between the indirect effect produced by routine infant/childhood immunization and the direct effect of immunizing the adults.
The Pneumococcal Work Group presented a variety of studies on this issue to include a case control study that examined PCV13 effectiveness among adults 65 years old and older, a report of the direct and indirect effects among adults greater than 65 years old, a report of the effectiveness of PCV13 in preventing pneumococcal pneumonia among U.S. adults along with coverage estimates, and an estimate of the burden of pneumococcal pneumonia among adults in the U.S. and an outline of research that is planned for the future that will help to inform potential policy change.
At upcoming meetings, the ACIP will continue to receive updates about PCV13 and the impact it is having on pneumonia, as well as a model estimating public health impact and cost-effectiveness of different policy options for adults 65 years of age and older, including eliminating PCV13 or expanding vaccine indications.
Vaccines for Prevention and Treatment of Healthcare Associated Infections
The Committee received a presentation on the role of vaccines in the treatment and prevention of healthcare associated infections, including how vaccines present an opportunity to reduce antibiotic use. A new work group has been established to review this area and to update existing recommendations.
Meningococcal
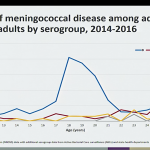
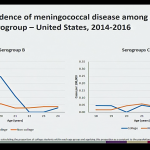
This session of the ACIP included a review of the epidemiology of meningococcal disease among college students from 2014-2016, which highlighted the following:
- In the 1990’s meningococcal disease and outbreaks in young adults were due primarily to serogroup C.
- In 2005, the ACIP recommended routine vaccination with the quadrivalent meningococcal conjugate known as MenACWY at ages 11-12 and among unvaccinated college freshman living in residence halls.
- In 2010, a booster dose was recommended at age 16.
- In the past 20 years, the overall incidence of meningococcal disease has declined 10-fold.
- Serogroup B is now the primary cause of meningococcal disease and outbreaks in young adults.
There are 2 MenB vaccines currently licensed in the U.S. In 2015, the ACIP recommended that a MenB vaccine series may be administered to adolescents and young adults age 16-23 years to provide short-term protection against most strains of serogroup B meningococcal disease, with a preferred age between 16-18 years of age. (This was known as a Category B recommendation.) At the time, the data demonstrated that the estimated incidence of serogroup B meningococcal disease among college students age 18-23 years was low (0.09 cases/100,000) which was similar to that of non-college students.
With improved an enhanced surveillance activities conducted in 45 states representing 98% of the population, additional data has since become available and revealed the following:
- Among the 1,178 cases of meningococcal disease reported during 2014-2016, 166 cases (14.1%) occurred in persons aged 18-24 years. Of those cases, approximately 51.2% occurred in college students and 48.8% in non-college students.
- Incidence of serogroup B meningococcal disease in college students is low, with an average of 20 cases and 2-4 outbreaks reported annually, however college students age 18-21 are at an increased risk compared to non-college students. Outbreaks have been an important factor, though it appears the risk remains elevated among college students even when excluding outbreak-associated cases.
- Incidence of serogroups C,W, and Y in this age group are lower and similar among college and non-college students, likely in part due to the adolescent MenACWY vaccination recommendation.
The ongoing challenges are complicated by the effectiveness and duration of protection from MenB vaccines. Data in adolescents suggests waning of antibodies as early as 12 months after completion of the primary MenB vaccine series. Additionally, evaluations show little-to-no impact of MenB vaccines on serogroup B carriage, suggesting that herd protection is unlikely. While MenB vaccines are expected to cover a wide range of circulating strains in the U.S., they will not prevent all cases. The number of vaccinated individuals needed to prevent a case are high.
Not surprisingly, there are gaps in awareness of MenB vaccines among parents and providers. Only 43% of parents of adolescents aged 16-19 report being aware of MenB vaccines, and uptake is estimated to be low at less than 10% of 16-18 year olds. Uptake in college students is unknown, however only 2% of colleges require MenB vaccine and 24% stock MenB vaccine.
The Work Group conclusions were as follows:
- Although the risk of serogroup B meningococcal disease is increased among college students, the number of preventable cases is low and the number needed to vaccinate to prevent a case is high.
- Limited duration of protection of MenB vaccines and the lack of evidence for impact on carriage may limit the ability to protect college students through the period of greatest risk, which is why MenB vaccination should occur as close to college entry as possible.
- While achieving high MenB coverage during college outbreaks has been challenging, routine vaccination of all college students would also be difficult, especially because awareness and uptake of MenB vaccines is currently low.
- The Work Group did not propose any changes to the current MenB vaccine recommendations, but suggested that the CDC could provide more guidance around clinical decision making during pre-college health visits.
Japanese Encephalitis
The Work Group presented a review of the disease and its current focus on cost-effective analysis.
Vaccine Supply
Due to large outbreaks of Hepatitis A among adults in several U.S. cities in 2017, there has been an increased demand for Hepatitis A vaccine which has resulted in constrained supplies of vaccine. CDC staff is carefully monitoring ongoing demand and usage, and has taken specific actions in conjunction with public health officials, manufacturers and the public sector to support the management of ongoing and future outbreaks, as well as routine vaccination.
It was announced that Merck is not currently distributing its pediatric Hepatitis B vaccine, but anticipates resuming distribution in early April, 2018. Merck is also not distributing adult Hepatitis B vaccine and does not intent to distribute the vaccine through the end of 2018. However, the Committee was informed that another manufacturer (GSK) has sufficient supplies of both the adult and pediatric Hepatitis B vaccines to address the anticipated gap, though preferences for a vial versus a syringe may not be met.
To learn more about vaccines supply and shortages, visit http://www.cdc.gove/vaccines/vac-gen/shortages/default.htm
To learn more about the topics discussed and the impact of these votes, join William Schaffner, MD, NFID Medical Director and liaison to the Advisory Committee on Immunization Practices (ACIP), and Amanda C. Cohn, MD, MPH, ACIP Executive Secretary, on a free webinar hosted by the National Foundation of Infectious Diseases (NFID) at 12:00 PM ET on 3/7/18 entitled “Updates from February 2018 ACIP Meeting”. Click here to register.
The next meeting of ACIP is scheduled for June 20-21, 2018.
Related Posts
The Public Health Emergency (PHE) declaration is ending on May 11, but COVID remains a threat. The PHE was first declared in 2020 in response to the spread of COVID-19 to allow for special...
This post was originally published with MediaPlanet in the FutureOfPersonalHealth.com Winter Wellness Issue, and was written by Vaccinate Your Family. Are you more likely to get sick during the winter? Yep – more viruses...

Leave a Reply